Do you know that electrolysis process is used widely in industries?
These are some common inductrial application of electrolysis are
- extraction of reactive metals
- purification of metals
- electroplating of metals
Metals that are very reactive (placed at the top position of the electrochemical series) such as sodium, calcium, magnesium and aluminium are extracted from their compounds using electrolysis. Electrolysis is usedbecause these reactive metals cannot be extracted from the minerals by reduction using carbon.
Extraction of Aluminium Metal from the Mineral Bauxite.
Bauxite is the major ore of aluminium consisting of Aluminium oxide, Al2O3. Cryolite (Na3AlF6) is added to aluminium oxide to lower its melting point from 2000˚C to about 950˚C. Molten aluminium oxide is electrolysed using carbon as electrodes in the electrolytic cell as show in figure below:

PURIFICATION OF METAL
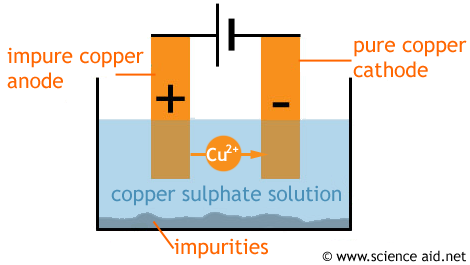
Impure metals containing impurities can be purified using electrolysis where the impure metal is used as the anode and a piece of metal is used as the cathode. the electrolyte is a solution containing the ions of the metal to be purified.
Purification of copper
Pure copper and silver can be obtained through the process of electrolysis. The impure copper is made to be anode while the cathode is a thin layer of pure copper.
ELECTROPLATING OF METAL
Electroplating is a process carried out to coat the surface of metal objects with thin and even layer of another metal. A more expansive or attractive metal such as silver or gold is coated onto the object to make it look more attractive and prevent corrosion.

.jpg)

