Do you know that electrolysis process is used widely in industries?
These are some common inductrial application of electrolysis are
- extraction of reactive metals
- purification of metals
- electroplating of metals
Metals that are very reactive (placed at the top position of the electrochemical series) such as sodium, calcium, magnesium and aluminium are extracted from their compounds using electrolysis. Electrolysis is usedbecause these reactive metals cannot be extracted from the minerals by reduction using carbon.
Extraction of Aluminium Metal from the Mineral Bauxite.
Bauxite is the major ore of aluminium consisting of Aluminium oxide, Al2O3. Cryolite (Na3AlF6) is added to aluminium oxide to lower its melting point from 2000˚C to about 950˚C. Molten aluminium oxide is electrolysed using carbon as electrodes in the electrolytic cell as show in figure below:

PURIFICATION OF METAL
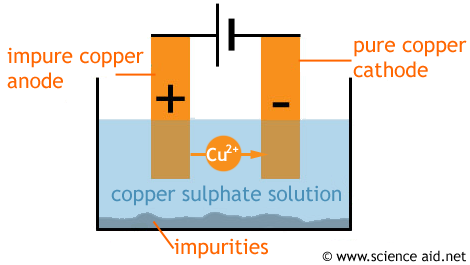
Impure metals containing impurities can be purified using electrolysis where the impure metal is used as the anode and a piece of metal is used as the cathode. the electrolyte is a solution containing the ions of the metal to be purified.
Purification of copper
Pure copper and silver can be obtained through the process of electrolysis. The impure copper is made to be anode while the cathode is a thin layer of pure copper.
ELECTROPLATING OF METAL
Electroplating is a process carried out to coat the surface of metal objects with thin and even layer of another metal. A more expansive or attractive metal such as silver or gold is coated onto the object to make it look more attractive and prevent corrosion.



The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal. Electroplating is primarily used for depositing a layer of material to bestow a desired property (e.g., abrasion and wear resistance, corrosion protection, lubricity, aesthetic qualities, etc.) to a surface that otherwise lacks that property. Another application uses electroplating to build up thickness on undersized parts.
ReplyDeleteThe process used in electroplating is called electrodeposition. It is analogous to a galvanic cell acting in reverse. The part to be plated is the cathode of the circuit. In one technique, the anode is made of the metal to be plated on the part. Both components are immersed in a solution called an electrolyte containing one or more dissolved metal salts as well as other ions that permit the flow of electricity. A power supply supplies a direct current to the anode, oxidizing the metal atoms that comprise it and allowing them to dissolve in the solution. At the cathode, the dissolved metal ions in the electrolyte solution are reduced at the interface between the solution and the cathode, such that they "plate out" onto the cathode. The rate at which the anode is dissolved is equal to the rate at which the cathode is plated, vis-a-vis the current flowing through the circuit. In this manner, the ions in the electrolyte bath are continuously replenished by the anode.[1]
ReplyDeleteOther electroplating processes may use a non-consumable anode such as lead. In these techniques, ions of the metal to be plated must be periodically replenished in the bath as they are drawn out of the solution
The anode and cathode in the electroplating cell are both connected to an external supply of direct current — a battery or, more commonly, a rectifier. The anode is connected to the positive terminal of the supply, and the cathode (article to be plated) is connected to the negative terminal. When the external power supply is switched on, the metal at the anode is oxidized from the zero valence state to form cations with a positive charge. These cations associate with the anions in the solution. The cations are reduced at the cathode to deposit in the metallic, zero valence state. For example, in an acid solution, copper is oxidized at the anode to Cu2+ by losing two electrons. The Cu2+ associates with the anion SO42- in the solution to form copper sulfate. At the cathode, the Cu2+ is reduced to metallic copper by gaining two electrons. The result is the effective transfer of copper from the anode source to a plate covering the cathode.
ReplyDeleteEffects
ReplyDeleteElectroplating changes the chemical, physical, and mechanical properties of the workpiece. An example of a chemical change is when nickel plating improves corrosion resistance. An example of a physical change is a change in the outward appearance. An example of a mechanical change is a change in tensile strength or surface hardness which is a required attribute in tooling industry
Electrochemistry has vast applications in the field of industry in modern times, based on the undergoing revolutions in the world of Science! One major one that hadn't been highlighted is in the making of electrochemical cells
ReplyDeleteIn the context of employing electrochemistry in metal purification, the process is entirely based on the anode cathode interplay with the metal to be purified being dipped in a solution of it's ions , and it acts as the anode! Another piece of metal of almost equal ranking in the electrochemical series is too dipped in the solution and used as the cathode!.
ReplyDeleteThe anode wears out in a solution of its ions,in what's commonly known as oxidation! Its oxidised and goes into solution of its ions, and the metal obtained is very pure
When we purchase our homes and visualize our belongings in the space, it seems there is a place for everything and more space than we had before. After we move in, we find there are a few things that can be improved. Before you tackle any home improvement project, you should plan it. Buy Adobe Photoshop CC 2020
ReplyDeleteI recently came across your blog and have been reading along. I thought I could leave my first comment. I don’t know what to say except that I have enjoyed scaning what you all have to say driveworks pro
ReplyDeleteThere are a handful of intriguing points with time here but I do not know if I see these people center to heart. There is certainly some validity but I’ll take hold opinion until I take a look at it further. Good post , thanks and we want a lot more! Added to FeedBurner also https://prosoftstore.com/
ReplyDeleteI am curious to find out what blog system you happen to be working with? I’m having some minor security problems with my latest blog and I’d like to find something more safe. Do you have any recommendations? 먹튀디비
ReplyDeleteThe advancement of new technology has been taking place since the beginning of human history. The question: are the impacts positive or negative? see here
ReplyDelete